Abstract
Introduction: Pegaspargase (Oncaspar, PEG-ASP) has replaced native E.coli asparaginase (L-ASP) in the first-line treatment for acute lymphoblastic leukemia (ALL) in many places because of its longer half-life and lower immunogenicity. Risk factors for allergic reactions to PEG-ASP remain unclear. Besides L-ASP, polyethylene glycol (PEG) is also a potential allergen, but the contribution of antibodies directed against PEG vs. L-ASP antigens has not been well characterized in front-line ALL trials. Herein, we assessed the usefulness of serum antibodies for diagnosing allergic reactions to PEG-ASP and to identify risk factors in a front-line ALL trial featuring intensive PEG-ASP treatment.
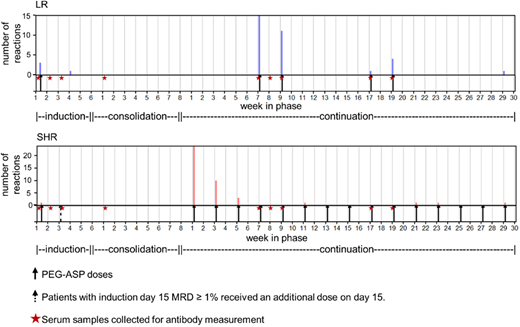
Methods: Of 600 patients enrolled in St. Jude's Total XVI study, 599 were evaluable for reactions to asparaginase and received either low-risk (LR) or standard/high-risk (SHR) therapy based on their risk for relapse. All patients received PEG-ASP at 3000 U/m2 on day 3 of remission induction; those with minimal residual disease (MRD) ≥ 1% on day 15 of induction received an additional dose of PEG-ASP. After induction, patients were randomized to receive PEG-ASP at 2500 U/m2 or 3500 U/m2 once every other week starting from week 1 of continuation treatment (SHR arm) or reinduction 1 (LR arm) (Figure 1). Reactions were graded using CTCAE v3.0. Nine serum samples per patient were scheduled on induction days 1, 8 and 15, consolidation day 1, and day 1 of continuation weeks 7, 8, 9, 17, and 19. Serum samples (n=5369) were analyzed for anti-PEG-ASP IgG by ELISA; positive samples were tested separately for anti-PEG and anti-L-ASP. Patient- and treatment-related variables were identified as potential risk factors for reactions and anti-PEG-ASP by χ2 test or multiple linear regression.
Results: Eighty-one (13.5%) patients developed grade 2-4 reactions to PEG-ASP; of these, 58 (72%) were grade 3/4. In univariate analysis, contrary to our prior front-line ALL trial using L-ASP (Liu, Leukemia 2012), there was no difference in the frequency of reactions between SHR (13.2%) and LR (13.8%) patients (P = 0.83). Patients with reactions received a median of only 3 doses of PEG-ASP before their reaction. The majority of reactions occurred upon the first few re-exposures to PEG-ASP following a 12- to 19-week hiatus after the induction doses, regardless of risk arm (P = 0.076): 72% of reactions among LR patients occurred during reinduction I, and 88% of reactions among SHR patients occurred during continuation weeks 1-6. Anti-PEG, not anti-L-ASP, was the predominant component of anti-PEG-ASP IgG (96% vs. 17%, P = 0.021), reflecting the antigenicity of PEG itself, as has been observed for other PEGylated drugs. Anti-L-ASP became a more common component of antibodies as therapy progressed (from 10% during induction to 36% at week 19 of continuation), whereas anti-PEG became a slightly less common component as therapy progressed (from 100% to 87%). Pre-treatment anti-PEG was present in 30 of 583 (5.1%) patients, more common in older patients (P = 2.1x10-5), and associated with a risk of subsequent reactions (P = 0.025), with 8 out of 30 developing reactions. Anti-PEG-ASP at all time points was positively associated (P ≤ 0.025 for days 1 & 15, consolidation day 1, continuation weeks 7, 8, 9, 17, & 19) with risk of a reaction during therapy except on induction day 8 (P = 0.24). For predicting future reactions, anti-PEG-ASP as early as consolidation day 1 had a sensitivity of 61% and a specificity of 90%, with an optimum receiver operating characteristic (ROC) curve at continuation week 7 (AUC = 0.85). In a multivariate analysis of clinical risk factors, only a lower number of intrathecal (IT) injections (P = 2.7x10-5) was predictive of reactions. Patients received 1 to 7 doses of IT therapy during induction: those who received more ITs (4-7 doses) had fewer reactions (7.6% of 301 patients) than those who received fewer (1-3 doses) ITs (20.2% of 248 patients); they were also less likely to have anti-PEG-ASP antibodies (P = 4.0x10-8).
Conclusions: Serum anti-PEG-ASP has good specificity and sensitivity for clinical allergic reactions to PEG-ASP treatment. Patients who received fewer IT injections during induction had a higher risk of allergy to PEG-ASP, possibly due to increased immunosuppression from multiple ITs. Anti-PEG, commonly observed pre-treatment, is likely the major mediator of PEG-ASP reactions and may be predictive of future reactions.
Inaba:Shire: Research Funding. Relling:Shire Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.